01. The definition and characteristics
Glucosyl steviol glycosides is a stevia-based flavor agent and flavor modifier used in food and beverage products. It is also known as enzyme-modified stevia.
Glucosyl steviol glycosides is a bioenzyme technology that selectively introduces the glucosyl into the stevioside molecule,increasing the amount of glycoside. Compared with stevioside, it has the advantages of reducing the natural bitterness and improving the solubility of stevia.
In the Japanese Pharmacopoeia, it is defined as: Enzymatic modified stevia is obtained by adding glucose to stevia extract using α-glucosyltransferase. It is composed of α-glucosyl stevioside,etc.
Domestic definition: Glucosyl steviol glycosides is made by using stevia leaves as raw material, extracted from stevia leaves by enzymatic glycosylation, then evaporation concentration and spray drying.
Glucosyl steviol glycosides is a white or light yellow powder with a cool and sweet taste. The sweetness is 100 to 150 times higher than that of white sugar. Glucosyl steviol glycosides overcomes the purity and bitter-aftertaste differences of common steviosides and has a better sweet taste. It is stable under usual food processing conditions. Its water dispersion is greatly increased by enzymatic techniques and, as a result, its higher solubility makes it ideal for a variety of beverages and wines. It is mainly used in low-calorie food, soft drinks, fruit juices, cold drinks,desserts, pickles and aquatic products.
02. Regulations in Japan and South Korea
Japan was one of the first countries to use enzyme modified stevia technology. Since the second half of the 1970s, stevia has been grown in Japan as an alternative to artificial sweeteners, and as the largest stevia use market, enzymatic modified steviosides have made slow but steady progress in the Japanese food industry, becoming widely used in a variety of food and beverage products. Glucosyl steviol glycosides is included in the Japanese Public Book of Food Additives.
In Korea, the use of enzyme modification of stevia was also in the 1979, currently the product is relatively mature. The related quality standards and testing methods of glucosyl steviol glycosides are included in the Korean Food Additives Catalogue.
03. European and American regulations
The approval of enzyme modified stevia in the United States was relatively late compared with the Japanese and Korean markets, but it developed rapidly. Since 2011, FDA has approved more than a dozen different models and specifications of enzyme modified steviosides as GARS, which can be used as sweeteners in a variety of food systems (See Figure Below). FEMA has approved multiple specifications of enzyme modified steviosides as food flavors since 2018, referring to FEMA numbers 4728, 4845, 4876, 4953 and others.
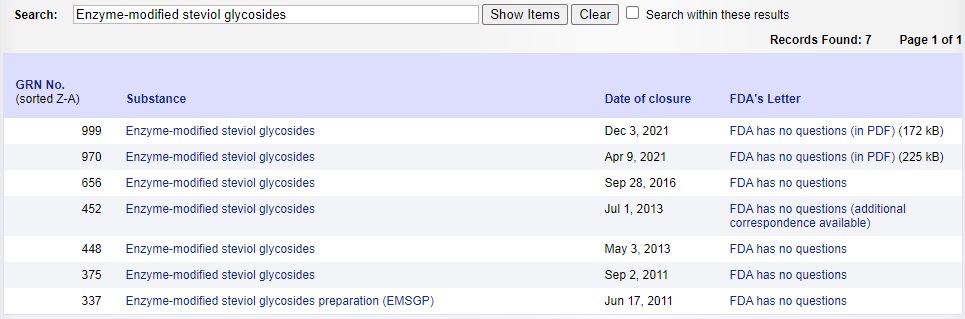
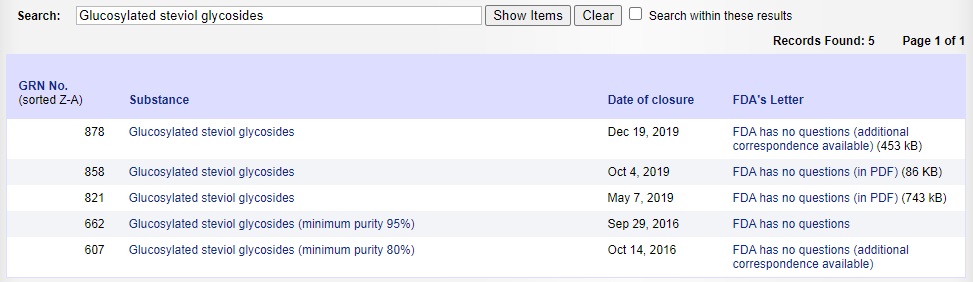
Image Source:FDA Official Website
The European food safety authority has repeatedly evaluating the security of glucosyl steviol glycosides as food additives in all kinds of food, and in February 2022 passed its safety assessment, assessment panel concluded that there are no safety concerns with the use of glucosyl steviol glycosides as a new food additive at recommended levels of use and use. The recommended use and levels of use are the same as those of the approved steviol glycosides (E 960a-960c) .
04. Chinese regulations
In China, according to the GB2760-2014 standard for the use of food additives and the Announcement No. 8 of 2016 of the National Health and Family Planning Commission of PRC, glucosyl steviol glycosides can be used as a food flavor of food additives, which can be used in all kinds of food except Table B.1 of GB2760-2014. Meanwhile the quality specification requirements of glucosyl steviol glycosides have been formulated.
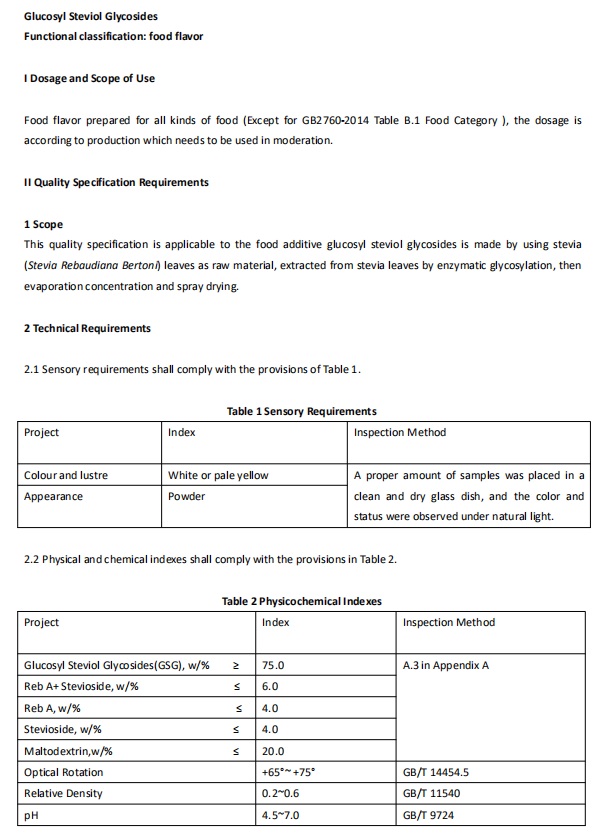
Image Source:FDA Official Website
L&P Food Ingredient Co., Ltd has been supplying glucosyl steviol glycosides since 2020.At present L&P Food Ingredient Co., Ltd can provide a variety of specifications and models of products, which provide high-quality solutions for international customers who have the needs of sugar reduction,natural and clean labels.